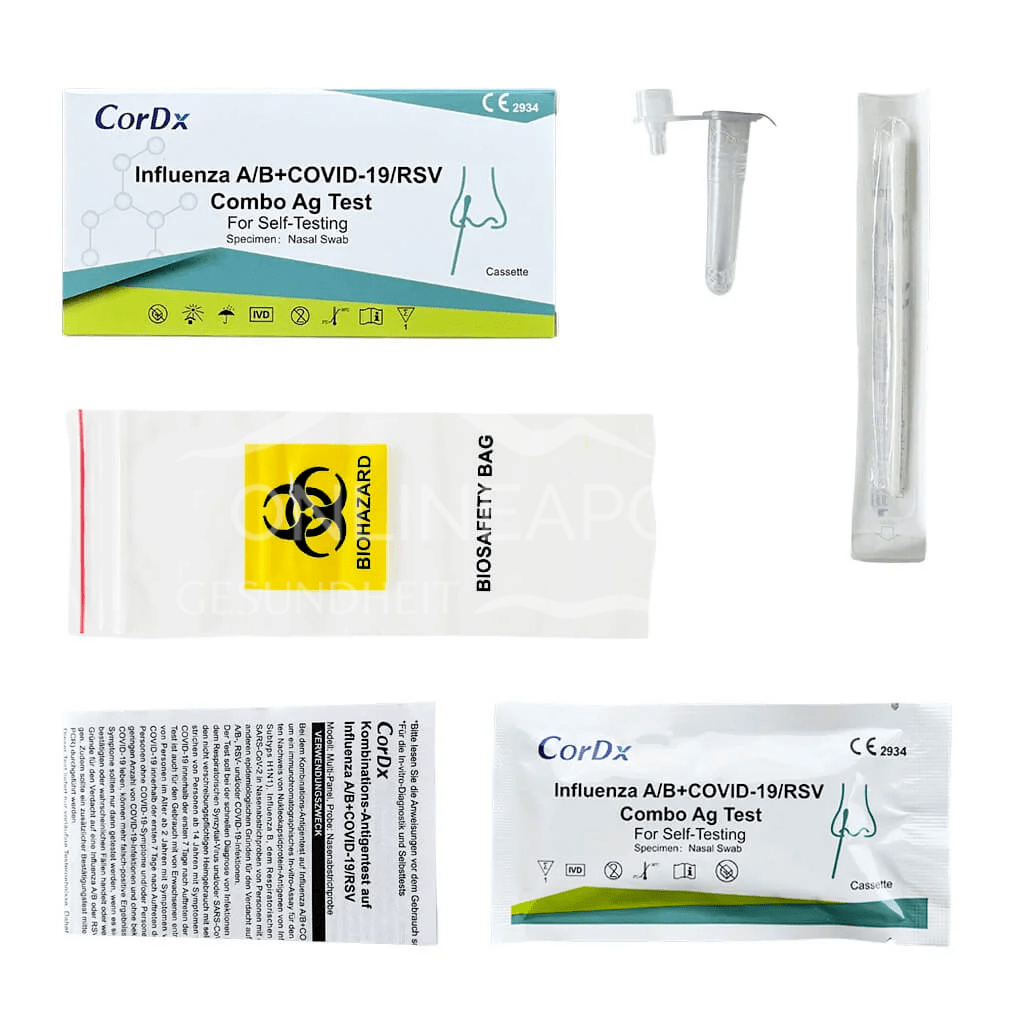
CorDx Influenza Test A + B, COVID-19, RSV, Combi Test 4in1 is an antigen test. Covid, influenza A/B and RSV can be detected within 15 minutes using a simple nasal swab.
€6.51*
Prices incl. VAT plus shipping costs (May vary by country)
Ready to ship in 1-3 working days ⁴
- Quick to perform: The test can be carried out in just a few minutes.
- 4in1 combination test: Covid, influenza A/B and RSV
- Evaluation after 15 minutes
- Easy to use: The test is simple to perform, just swab your nose.
- Storage at 2 - 30 °C CE certified clinically tested
- Includes all necessary utensils
- Influenza A: For influenza A antigen detection, the positive coincidence rate is 100.00%, the negative coincidence rate is 99.34% and the overall coincidence rate is 99.43%.
- Influenza B: When detecting the influenza B antigen, the positive coincidence rate is 96.00 %, the negative coincidence rate is 99.67 % and the overall coincidence rate is 99.15 %.
- RSV: For RSV antigen detection, the positive coincidence rate is 98.98%, the negative coincidence rate is 99.21% and the overall coincidence rate is 99.14%.
- Covid-19: For COVID-19 antigen detection, the positive coincidence rate is 89.09%, i.e. 98.67% for Ct≤25, 93.00% for Ct≤30, and the negative coincidence rate is 100.00%, the overall coincidence rate is 97.86%.
- Influenza A: 1.5×104 TCID50/mL
- Influenza B: 1.5×105 TCID50/mL
- Covid-19: 200 TCID50/mL
- RSV: 1.0×104 TCID50/mL
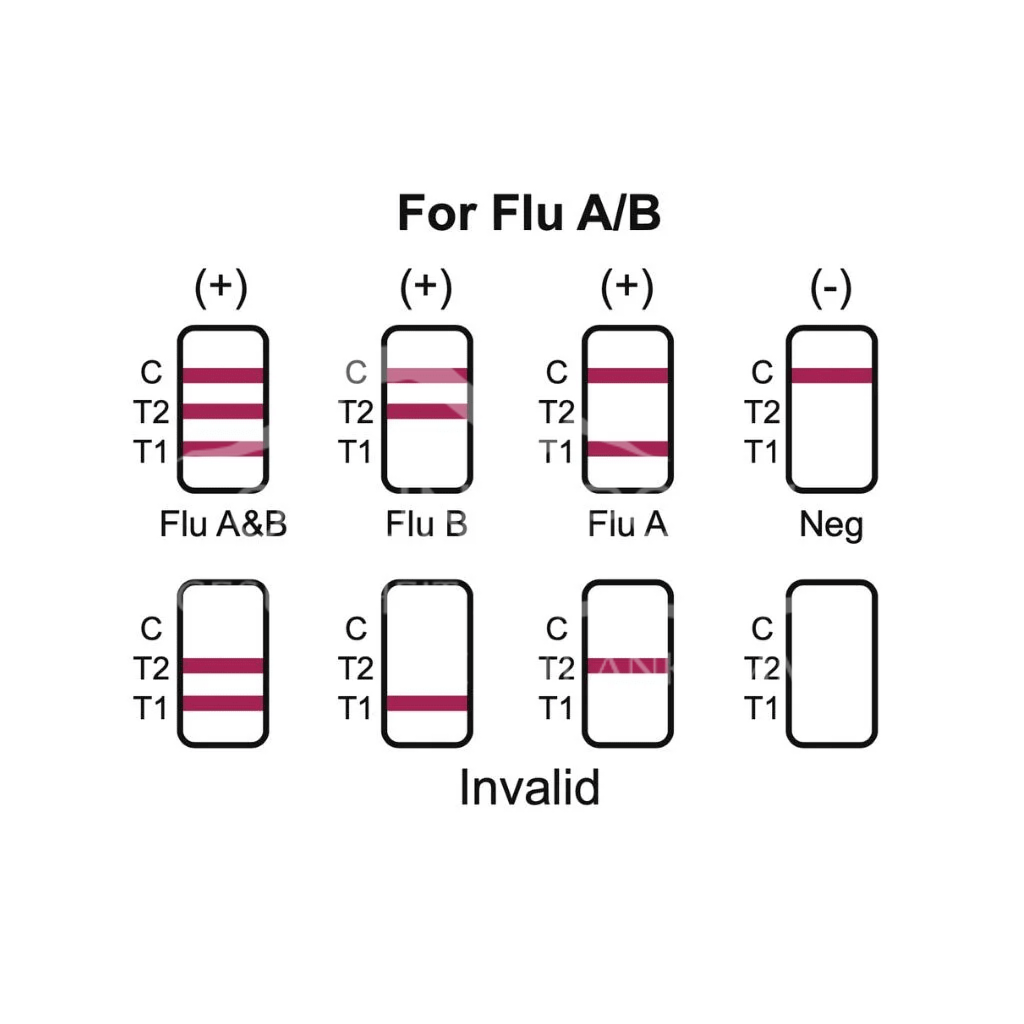
- The Influenza A, Influenza B Antigen Test Strip uses monoclonal mouse anti-influenza A antibodies (T1), monoclonal mouse anti-influenza B antibodies (T2) and polyclonal goat anti-mouse IgG antibodies (C), each immobilised on a nitrocellulose membrane. The test uses colloidal gold to label monoclonal mouse anti-influenza A antibodies and monoclonal mouse anti-influenza B antibodies. Nanocolloidal gold technology is used and highly specific antibody-antigen reactions and the principle of immunochromatographic analysis technology are applied. During testing, the influenza A antigens in the sample combine with the colloidal gold-labelled mouse anti-influenza A monoclonal antibody to form a complex, which then combines with the mouse anti-influenza A monoclonal antibody coated in the T1 test line during chromatography to form a red line in the T1 line.
- During testing, the influenza B antigens in the sample combine with the colloidal gold-labelled mouse anti-influenza B monoclonal antibody to form a complex, which then combines with the mouse anti-influenza B monoclonal antibody coated in the T2 test line during chromatography to form a red line in the T2 line. If the samples do not contain influenza type A and B antigens, no red lines will appear in the T1 and T2 areas. Regardless of the presence of influenza type A or B antigens in the sample, a red line will always appear in the quality control area (C) if possible. The red line in the quality control area (C) is used to check whether 1. a sufficient sample volume has been added, 2. a proper flow rate has been achieved, 3. and as a quality control for the reagents.
- The COVID-19, RSV Antigen Test Strip uses mouse monoclonal anti-COVID-19 antibody (T2), mouse monoclonal anti-RSV antibody (T1) and goat polyclonal anti-mouse IgG antibody (C), each immobilised on a nitrocellulose membrane. It uses colloidal gold to label the mouse monoclonal anti-COVID-19 antibody and the mouse monoclonal anti-RSV antibody. The nanocolloidal gold technology is used and highly specific antibody-antigen reactions and the principle of immunochromatographic analysis technology are applied. During testing, the COVID-19 antigen in the sample combines with the colloidal gold-labelled mouse monoclonal anti-COVID-19 antibody to form a complex, which was then combined with the mouse monoclonal anti-COVID-19 antibody coated in the T2 test line during chromatography; at this point, there is a red line in the T2 area (the test is positive).
- The RSV antigen in the sample combined with the RSV monoclonal antibody labelled with colloidal gold to form a complex, which was then combined with the mouse monoclonal anti-RSV antibody coated in the T1 test line during chromatography; at this time, there is a red line in the T1 area (The test is positive). If the samples do not contain COVID-19 and RSV antigens, there are no red lines in the T1 and T2 areas (the test is negative). Regardless of the presence of COVID-19 or RSV virus antigens in the sample, a red line always forms in the quality control area (C). The red line in the quality control area (C) is used to check whether 1. a sufficient sample volume has been added, 2. a proper flow rate has been achieved, and 3. as a quality control for the reagents. If this does not occur, the test must not be analysed.
Dosage form
Application
| Piece: | 1 |
|---|