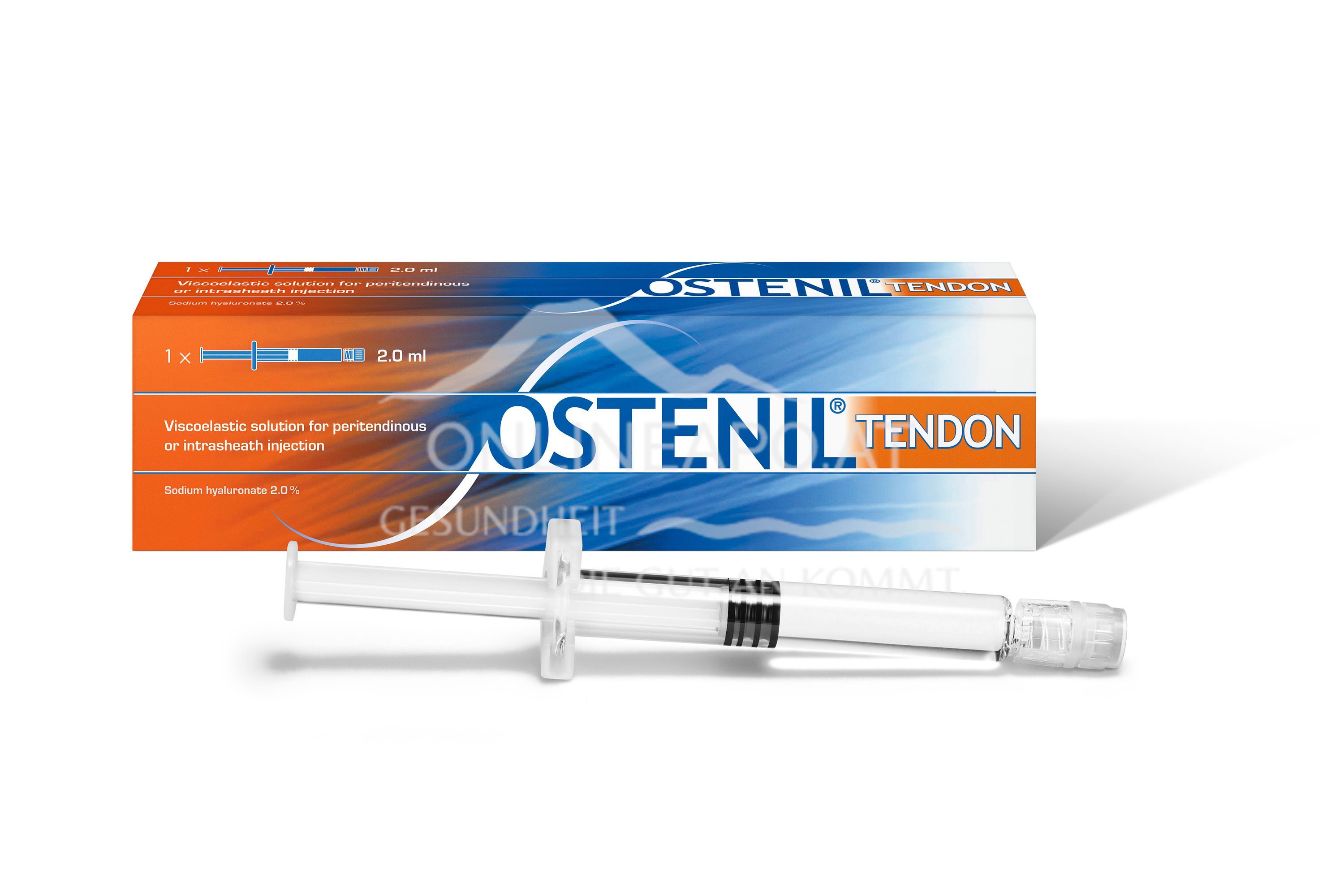
Ostenil® Tendon 40mg/2ml - Is a certified medical product. It contains highly concentrated hyaluronic acid (2%) and is used to treat pain and restricted mobility in tendon problems.
€95.64*
Prices incl. VAT plus shipping costs (May vary by country)
Ready to ship in 1-3 working days ⁴
OSTENIL® TENDON Sodium hyaluronate from fermentation 2.0%. Viscoelastic solution for peritendinous injection or for injection into the tendon sheath. Sterile due to moist heat.
Indications
- For the treatment of pain and restricted mobility in tendon complaints.
Properties and mode of action
- A tendon is a robust structure of fibrous connective tissue designed to transmit forces from muscle to bone and to withstand tension during muscle contraction. Tendons can be surrounded by different structures: e.g. fibrous ligaments, synovial sheaths, tendon sheaths, bursae. Overuse or incorrect loading can cause inflammation and/or degenerative changes in the tendon, leading to pain and loss of function. Making the tendon more lubricated could reduce pain, improve tendon function and reduce the possibility of adhesions.
- Due to its lubricating and viscoelastic properties, OSTENIL® TENDON supports tendon lubrication and the physiological regeneration process. In addition, the macromolecular structure of OSTENIL® TENDON reduces the free passage of pro-inflammatory cells and molecules through the tendon sheath.
- OSTENIL® TENDON is a clear solution of natural, highly pure sodium hyaluronate, which is obtained by fermentation and is therefore free from animal proteins. OSTENIL® TENDON is also stabilised by the addition of mannitol, a free radical scavenger. In biocompatibility studies, OSTENIL® TENDON has proven to be particularly well tolerated.
Dosage form
injection
How to use
- Inject OSTENIL® TENDON twice a week around the diseased tendon or into the diseased tendon sheath. Several tendons can be treated at the same time. Repeated treatments are possible if required.
- As long as the sterile packaging has not been opened, the contents and surface of the OSTENIL® TENDON prefilled syringe are sterile. The prefilled syringe is removed from the sterile packaging, the cap is removed from the Luer-Lock connection, a suitable cannula (e.g. 25 to 27 G) is attached and locked in place by turning it slightly. Before the injection, any air bubble is removed from the syringe.
Ingredients
1 ml isotonic solution contains 20.0 mg sodium hyaluronate and sodium chloride. Disodium hydrogen phosphate, sodium hydrogen phosphate, mannitol and water for injection.
| Piece: | 1 |
|---|