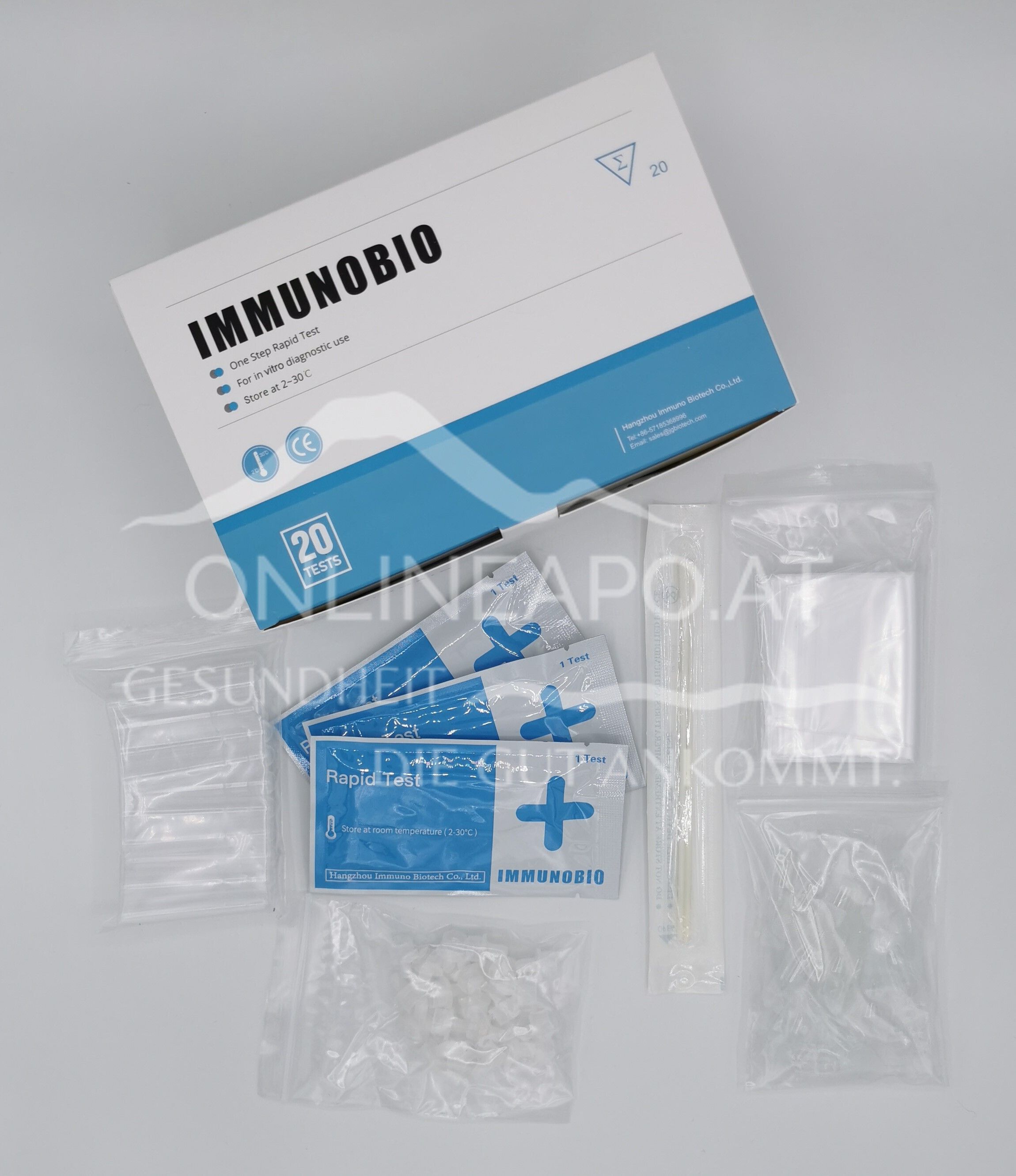
Immunobio Antigen Rapid Test 4 in 1 - The 4 in 1 Corona Rapid Test. Samples can be taken in 4 different ways: via saliva - saliva test or spit test, anterior nasal area, oropharynx and posterior nasopharynx.
| Quantity | Unit price | Base price |
|---|---|---|
| To 20 |
€3.39*
€6.99*
(51.5% saved)
|
€0.17* / 1 Stück |
| From 21 |
€3.19*
€6.49*
(50.85% saved)
|
€0.16* / 1 Stück |
Prices incl. VAT plus shipping costs (May vary by country)
No longer available
Immunobio Antigen Rapid Test 4 in 1 - The 4 in 1 Corona Rapid Test. Samples can be taken in 4 different ways: via saliva - saliva test or spit test, anterior nasal area, oropharynx and posterior nasopharynx.
The following samples can be taken with the Immunobio Antigen Rapid Test 4 in 1: saliva swab, anterior nasal swab, posterior nasal swab, pharyngeal swab. The immunochromatographic lateral flow test is used for the rapid and qualitative detection of antigens in saliva samples from people who are suspected of being positive, i.e. infected, by the healthcare provider within the first seven days after the onset of corona symptoms. In general, the antigen is detectable in upper respiratory tract samples during the acute phase of infection. The result is used to definitively identify the SARS-CoV-2 antigen.
- Sensitivity 94.39 %
- Specificity 97.67 %
- 4 different sampling methods possible:
- Easy to use
- Fast and reliable test results in just 15 minutes
- Test storage at room temperature
- All test components - including sterile swab - are included
- No cross-reactivity with human pathogenic coronaviruses (such as hCoV-229E, -HKU1, -NL63 or -OC43) or influenza viruses (such as influenza A/B)
- Product as Covid-19 In Vitro Diagnostics VAT exempt
- Recognises Omikron variant (according to Paul-Ehrlich-Institut evaluation)
How to use
- For personal detection of Covid-19 disease only. When used correctly, the result is 94.39% correct and indicates a negative or positive Covid-19 disease.
- The Immunobio Antigen Rapid Test is on the list of the Federal Office for Safety in Health Care (BASG). The BASG has classified this rapid antigen test as suitable for self-testing by private individuals.
- Antigen tests listed by BfArM (AT150/21) and evaluated by the Paul-Ehrlich-Institut (PEI)
- test cassette
- Sterilised swab (per test - 1x thin head/1x thick head)
- Extraction reagent 0.3ml
- Extraction tube incl. drip tip
- Collection bag for saliva samples
- Dropper (located with the test cassette)
- Package leaflet (German)
- There is an obligation to report positive test results in accordance with the Epidemics Act as well as negative results in accordance with the Laboratory Reporting Ordinance. (Tel. 1450)
- The result of a rapid antigen test is only a current snapshot. The test result is for your information only and is not suitable for submission to the authorities or your employer.
| Piece: | 20 |
|---|