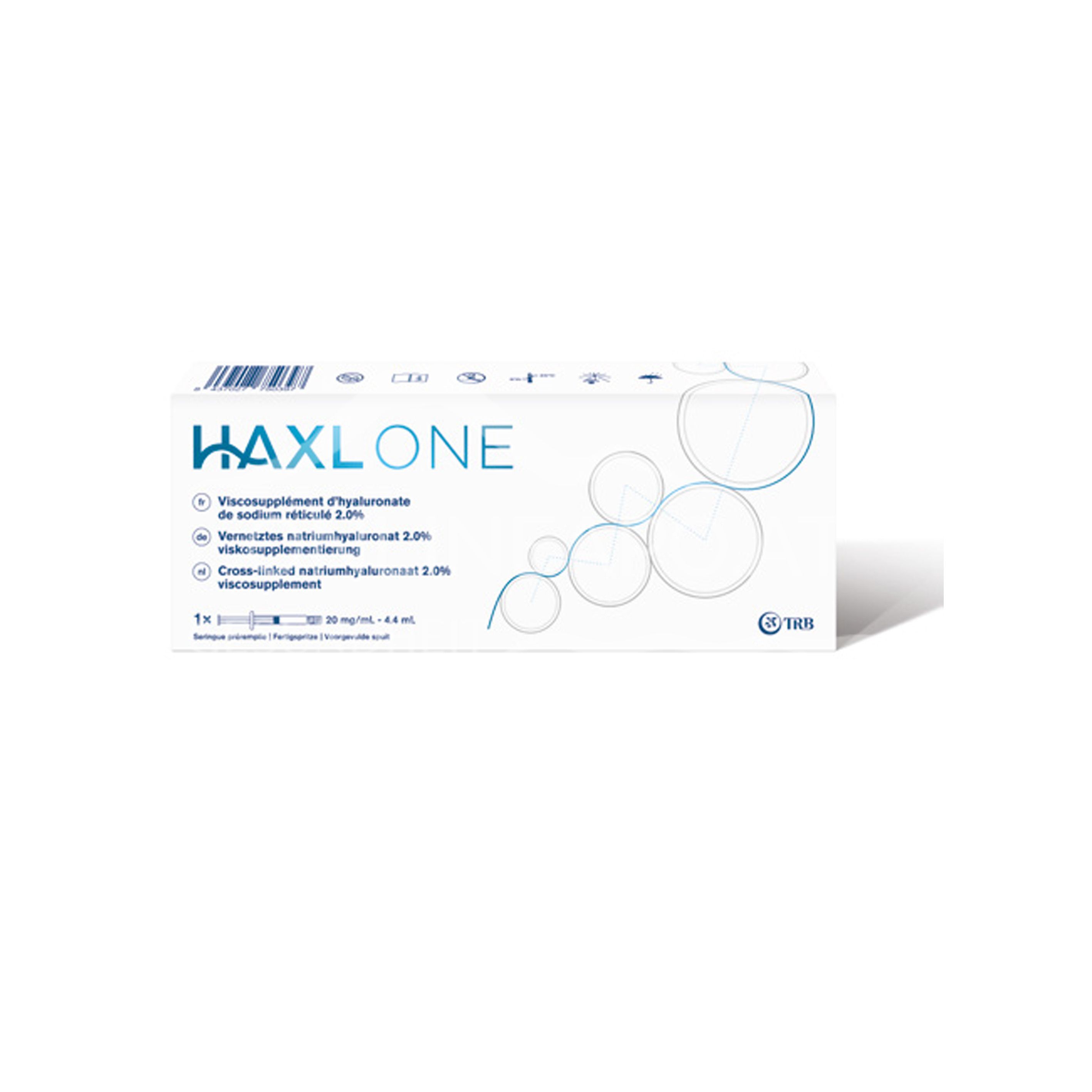
The HAXL ONE 20 mg/ml prefilled syringe for the intra-articular treatment of your knee osteoarthritis. High-purity, cross-linked sodium hyaluronate 2% for improved joint function and less pain. Class III medical device.
The HAXL ONE 20 mg/ml prefilled syringe for the intra-articular treatment of your knee osteoarthritis. High-purity, cross-linked sodium hyaluronate 2% for improved joint function and less pain. Class III medical device.
The disposable syringe for the treatment of knee osteoarthritis with cross-linked 2% hyaluronic acid
- Cross-linked hyaluronic acid as a single injection (one-shot treatment)
- Effective by relieving pain & improving mobility and quality of life
- Well tolerated as it does not contain any animal proteins
Hyaluronic acid therapy with HAXL ONE
HAXL ONE is a modern hyaluronic acid therapy that is injected specifically into the affected knee joint in a single injection (one-shot therapy). The hyaluronic acid in HAXL ONE has been cross-linked using the innovative SARE Technology® for a particularly stable structure with a high buffering effect and long-lasting joint lubrication.
The result: a targeted and effective therapythat noticeably relieves the knee joint - for more mobility and quality of life!
The special features of SARE Technology®
HAXL ONE is based on the innovative SARE Technology® and combines two special features: the hybridisation of different lengths of hyaluronic acid and the cross-linking of the individual molecules.
- Hybridisation: The combination of hyaluronic acid chains of different lengths results in a so-called hybrid structure. This molecular diversity enables optimum adaptation to the requirements of the knee joint.
- Cross-linking: Cross-linking connects the hyaluronic acid molecules with each other using a special compound. This creates a dense and uniform network that gives the gel a particularly stable structure. This structure makes the hyaluronic acid more resistant to degradation processes and supports both even lubrication and targeted cushioning of the knee joint.
Areas of application
HAXL One is intended for intra-articular injection into the synovial space of the knee joint to restore the viscosity of the synovial fluid in patients with osteoarthritis of the knee whose joint function is impaired by a loss of viscosity and who have responded inadequately to non-pharmacological measures and simple analgesics.
Dosage form
prefilled syringe
Directions for use
HAXL One must be administered by a qualified healthcare professional by intra-articular injection in accordance with all rules of aseptic procedure and injection technique. HAXL One is injected with an appropriate sterile needle. The manufacturer recommends the use of a 19G needle. The use of HAXL One by a single injection is recommended. The exact dose is at the discretion of the treating physician. Remains of the product must be disposed of properly.
- Before injection, the area to be treated must be carefully disinfected and the product brought to room temperature.
- Remove the sterile barrier and remove the syringe from the blister.
- Remove the Luer lock cap from the syringe without touching the tip so that it remains sterile.
- Attach a suitable sterile needle to the syringe. The manufacturer recommends the use of a 19G needle. Before use, check that the needle is correctly locked onto the Luer lock adapter of the syringe.
- If necessary, remove the air from the syringe.
- Inject into the synovial space of the affected knee joint, following aseptic injection procedures.
- Dispose of the used syringe and unused material after injection. The exact dose is at the discretion of the treating physician. The recommended dose is 3 - 4 ml for each knee joint.
Warnings & Precautions: HAXL One must be injected strictly intra-articularly, following the rules of aseptic injection techniques. The application must be carried out using sterile Luer-Lock needles. Do not inject into blood vessels or surrounding tissue. To avoid pain after the injection, it is recommended to rest the treated joint for 48 hours after the injection. The product must not be used if the packaging is damaged or the expiry date has been exceeded. The medical device is intended for single use only. Do not reuse as there is a risk of infection. Do not re-sterilise, resterilisation can affect the physico-chemical properties of the medical device and impair its effectiveness and safety. The used syringe and needle must be disposed of in an appropriate container. Keep out of the reach of children.
Ingredients
- HAXL One consists of highly purified sodium hyaluronate, pharmacopoeial grade salts and water for injection.
- Composition (mg/ml): Cross-linked sodium hyaluronate 20, disodium phosphate dodecahydrate 0.6, sodium dihydrogen phosphate dihydrate 0.05, sodium chloride 8, water for injections q.s..
| Piece: | 1 |
|---|